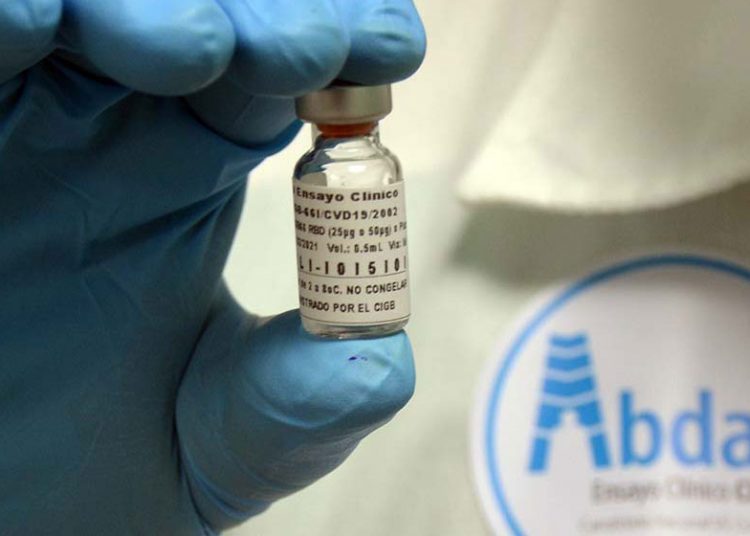
The Center for the State Control of Medicines, Equipment and Medical Devices (CECMED) granted Abdala 50 µg Emergency Use Authorization this Friday, turning it into the first COVID-19 vaccine developed in a Latin American country that receives such approval.
In accordance with the provisions of current regulations and provisions, once it was confirmed that the requirements and parameters demanded in terms of quality, safety and efficacy for this type of procedure have been met, the Cuban regulatory authority gave the go-ahead for vaccination on the island with the drug developed by the Center for Genetic Engineering and Biotechnology (CIGB), Cubadebate reported.
— CECMED (@cubacecmed) July 9, 2021
CECMED made the approval effective through Resolution No. 113 dated July 9, 2021, after “concluding a rigorous evaluation process of the file submitted” for the request of the Emergency Use Authorization and having carried out the inspections at the plants involved in the production process of the vaccine, the source indicated.
The regulatory entity assures that it confirmed the data obtained in the completed Phase I and Phase II clinical trials, in addition to the Phase III, still in progress, which has demonstrated an 92.28% efficacy in the prevention of symptomatic forms of the disease.
Cited by the source, CECMED adds that an adequate safety profile of Abdala was verified, supported by the number of doses applied to volunteers in the clinical trials, as well as in the intervention study with the drug in health and medical personnel and the health intervention in populations and territories at risk being carried out on the island.
Abdala has an efficacy of 92.28% with a scheme of three applied doses, according to clinical studies of the immunogen, released in June by the CIGB.
Candidato vacunal cubano Soberana 02 muestra eficacia del 62 % en ensayos clínicos
With these results, Abdala will be officially considered a COVID-19 vaccine, since the requirements demanded by the World Health Organization (WHO) for its approval establish a 50% efficiency for the registration of vaccines and for emergency use authorization.
“Abdala’s effectiveness with three doses will be an event that will multiply pride,” Cuban President Miguel Díaz-Canel tweeted at the time, a little before the news was released.